Applications and Analogies |
|
edited by
Ron DeLorenzo
Middle Georgia College
Cochran, GA 31014 |
Vyacheslav V. Samoshin*
Lomonosov Moscow State Academy of Fine Chemical Technology, Vernadsky Prospect 86, Moscow, 117571, Russia
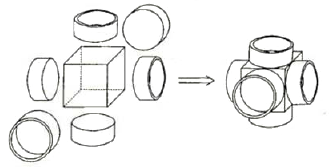
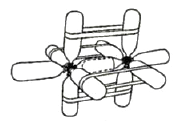
CPK or Dreiding molecular models do not allow for demonstration of shape and direction of atomic and molecular orbitals, their overlaps, interactions, and hybridizations. These may be imitated by means of molecular models easily constructed from plastic soda bottles (Fig. 1-4). For such models one may attach the screw caps to die faces of wooden or plastic tetrahedrons, trigonal prisms, or cubes (by screws, glue, wire; see Fig. 5). The use of wire gives the most solid models. When the bottles are screwed into the lids, the models of sp3, sp1, or sp hybridized atoms are obtained (Figs. 1-4). However, the tetrahedral geometry may be reproduced in a simpler way:
* On leave at University of the Pacific, Stockton, CA 95211. |
|
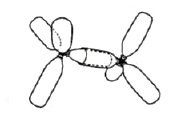
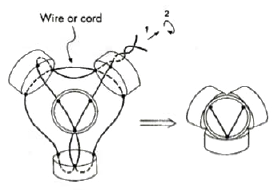
a wire or cord is passed through three equidistant holes in the top edge of each of four lids, and is tightened (Fig. 6). Using bottles of various shapes, sizes, and colors, one may visualize "hybrid" or "p" orbitals and "lone electron pairs", and construct large models for lecture demonstrations or smaller ones for seminars and students'lab exercises. The orbital overlap leading to a bond formation is imitated by insertion of a smaller bottle into a larger one with the bottom cut off (Figs. 2-4). Rubber or plastic rings put on pairs of "p orbitals" mimic π bond formations {Figs. 3, 4).
The models described above arc convenient for demonstrations and explanation of important chemical and stereo-chemical concepts. |
 |
|
 |
| Figure 1. The model of sp3 hybrid orbitals (methane). |
Figure 4. The model of triple bond (acetylene). |
 |
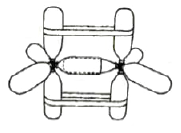 |
| Figure 2. The model of carbon-carbon a bond (ethane). |
Figure 5. The construction of an sp hybridized atom. |
 |
 |
| Figure 3. The model of double bond (ethylene). |
Figure 6. The construction of a tetrahedral atom. |
| JChemEd.chem.wisc.edu Х Vol.75 No. 8 August 1998 Х Journal of Chemical Education |